Biologics Contract Manufacturing Market to Reach US$25.1Bn by 2033 at 7.9% CAGR | Persistence Market Resaerch
The biologics contract manufacturing market is rapidly growing, driven by outsourcing, advanced therapies, and expanding global biopharmaceutical demand.
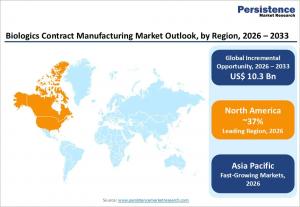
LONDON, UNITED KINGDOM, February 16, 2026 /EINPresswire.com/ -- The global biologics contract manufacturing market is witnessing remarkable growth, projected to reach US$ 25.1 billion by 2033 from an estimated US$ 14.8 billion in 2026, expanding at a compound annual growth rate (CAGR) of 7.9% between 2026 and 2033. This growth trajectory is primarily driven by the surge in demand for complex biologic therapeutics, including monoclonal antibodies (mAbs), recombinant proteins, and cell and gene therapies, alongside a strategic industry shift toward outsourcing manufacturing to specialized Contract Development and Manufacturing Organizations (CDMOs).
The U.S. Food and Drug Administration (FDA) approved a record 50 novel drugs in 2024, 16 of which were biologics, marking the highest number of mAbs approvals since 2015. This underscores the accelerating biologics pipeline that requires expanded external manufacturing capacity. Concurrently, the adoption of advanced production technologies, such as single-use systems, continuous bioprocessing, and automation, has enhanced efficiency and reduced contamination risks, making outsourcing increasingly appealing for pharmaceutical companies seeking cost-effective, compliant manufacturing solutions.
Download Your Free Sample & Explore Key Insights: https://www.persistencemarketresearch.com/samples/31928
Key Market Highlights
North America is the market leader, accounting for 37% of the global share in 2025, driven by regulatory expertise, proximity to major pharmaceutical headquarters, and a strong biopharmaceutical innovation ecosystem. Asia-Pacific is the fastest-growing region, with a projected CAGR exceeding 13% through 2030, fueled by initiatives such as China’s Healthy China 2030 program, India’s emergence as a cost-competitive manufacturing hub, and South Korea’s investment in advanced infrastructure.
Monoclonal antibodies dominate the product segment with 24% market share in 2025, supported by 13 FDA approvals in 2024 alone. Oncology represents the fastest-growing therapeutic area, driven by the increasing incidence of cancer, the aging global population, and the rising adoption of targeted therapies such as bispecific antibodies, checkpoint inhibitors, and antibody-drug conjugates. The global monoclonal antibody market is projected to exceed US$ 823 billion by 2034, highlighting the sustained demand for contract manufacturing services.
Market Dynamics
Growth Drivers
Escalating Pipeline of Biologic Approvals
The industry is witnessing a shift from small-molecule drugs to complex biologics, creating unprecedented demand for specialized manufacturing. Over 305 CDMOs are engaged in biologics production, with more than 90% offering finished dosage form services. The need for metric-ton scale production was highlighted during the COVID-19 pandemic, when global mAb manufacturing capacity proved insufficient. Consequently, pharmaceutical companies are increasingly establishing long-term partnerships with CDMOs to ensure scalable production without large capital investments.
Strategic Outsourcing for Cost and Operational Efficiency
Outsourcing biologics manufacturing allows companies to mitigate high infrastructure costs, reduce facility construction timelines, and focus capital on research and development. Data shows an 11% average growth in outsourcing budgets in 2025, up from 8.5% in 2024. By leveraging CDMO expertise, companies can cut infrastructure costs by 40–50% and accelerate commercialization timelines, particularly benefiting small and mid-sized biotechnology firms lacking internal manufacturing capacity.
Get Custom Insights Designed for Your Business: https://www.persistencemarketresearch.com/request-customization/31928
Market Restraints
Stringent Regulatory Compliance
Biologics manufacturing is subject to rigorous regulatory standards from the FDA, EMA, ICH, and regional authorities, creating operational challenges. Compliance with cGMP, analytical testing, and quality control increases costs and extends facility commissioning timelines by 6–12 months. Continuous updates to regulatory guidelines, particularly for Advanced Therapy Medicinal Products (ATMPs), necessitate ongoing investment in facility upgrades and staff training, posing challenges for smaller CDMOs.
Intellectual Property and Technology Transfer Risks
Outsourcing biologics manufacturing involves sharing proprietary processes and cell line technologies, raising IP protection concerns. In regions with uncertain IP enforcement, pharmaceutical companies face risks of misappropriation or competitive leakage, delaying partnerships by 4–8 months. This is particularly significant for high-value biologics, where protection of trade secrets is critical.
Category Insights
Product Analysis
Monoclonal antibodies lead the market due to their expanding applications in oncology, autoimmune, and infectious diseases. Other biologics, including recombinant proteins and cell and gene therapies, contribute to market growth through diversified therapeutic applications.
Therapeutic Area Analysis
Oncology drives demand for contract manufacturing, supported by FDA approvals of 6 mAbs in 2024 for cancer indications. Therapies such as checkpoint inhibitors, HER2-targeting antibodies, and bispecific T-cell engagers are creating sustained capacity requirements, with CDMOs specializing in oncology able to command premium pricing.
End User Analysis
Large pharmaceutical companies dominate as end-users, representing over 80% of strategic initiatives. Partnerships with CDMOs, such as Samsung Biologics’ collaborations with 17 of the top 20 pharma companies, enable companies to manage peak production periods, diversify supply chains, and focus on R&D investment.
Regional Insights
North America remains the leader due to its established biopharmaceutical infrastructure and regulatory expertise. Asia-Pacific is emerging as a manufacturing powerhouse, with China, India, and South Korea investing heavily in capacity and infrastructure, positioning the region for substantial market share gains through 2033.
Competitive Landscape
The market is highly competitive, featuring large, mid-sized, and specialized CDMOs offering end-to-end solutions. Key players include Samsung Biologics, WuXi Biologics, Lonza Group AG, Fujifilm Diosynth Biotechnologies, BioXcellence (Boehringer Ingelheim), and AbbVie CM (AbbVie Inc.). Companies are expanding capacity, adopting innovative manufacturing technologies, and leveraging regulatory expertise to secure market leadership.
Checkout Now & Download Complete Market Report: https://www.persistencemarketresearch.com/checkout/31928
Market Segmentation
Product
Monoclonal Antibodies
Recombinant Proteins
Vaccines
Insulin
Interferons
Growth Factors
Others
Therapeutic Area
Oncology
Autoimmune Disease
Metabolic Disease
Ophthalmology
Cardiovascular Disease
Infectious Disease
Neurology
Respiratory Disorder
Others
Application
Hybrid Electric Vehicles (HEVs)
Consumer Electronics
Medical Devices
Industrial Equipment
Power Tools
Emergency Lighting & Backup Power
End User
Pharmaceutical Companies
Biotechnology Companies
Academic & Research Institutes / CROs
Others
Regions
North America
Europe
East Asia
South Asia
Read Related Reports:
Autologous Conditioned Plasma Market: The autologous conditioned plasma market to reach US$1.86 billion by 2033, driven by growing use of orthobiologic therapies and rising demand for regenerative treatments.
Pulmonary Fibrosis Treatment Market: The onychomycosis treatment market will grow from $4.1 Bn in 2025 to $5.8 Bn by 2032, supported by a steady 5.1% CAGR and rising demand for effective therapies.
Persistence Market Research
Persistence Market Research Pvt Ltd
+1 646-878-6329
email us here
Visit us on social media:
LinkedIn
Instagram
Facebook
YouTube
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.